The desire for permanent cosmetic change of eye color has driven the development of vari- ous surgical techniques aimed at achieving this transformation, pursuing more enduring solu- tions. This demand has led to the emergence of three primary surgical approaches: cosmetic iris implants, laser iris depigmentation, and cos- metic keratopigmentation (KTP). Each technique presents distinct advantages, yet also entails spe- cific limitations and potential risks. Cosmetic iris implants, originally designed for congenital or traumatic iris defects, have been repurposed for aesthetic use. However, they come with severe risks, including glaucoma, corneal endothelial cell loss, and even permanent vision impair- ment. As a result of these complications, they are not approved by major regulatory bodies and are widely considered unsafe. Laser iris depig- mentation offers a less invasive approach, using a Q-switched Nd:YAG laser to remove melanin from the anterior iris stroma. While it provides a natural-looking result, it lacks customization and has potential complications like patchy pigmentation, photophobia, and temporary intraocular pressure spikes. Additionally, there is limited long-term data on its safety. Cosmetic KTP, an advanced version of corneal tattooing, has emerged as the safest and most effective option. It involves embedding micronized min- eral pigments into the cornea, allowing for pre- cise, customizable, and long-lasting results. Stud- ies show high patient satisfaction and minimal risks when properly performed. Among these techniques, KTP appears to be the best choice owing to its safety and aesthetic flexibility, while cosmetic iris implants should be avoided because of their high risk of complications, and laser iris depigmentation deals with limitations in color selection and long-term reliability. While KTP currently seems the safest option for cosmetic eye color change, this is largely based on limited single-center data and should be confirmed by larger studies in the future.
| Key Summary Points |
| Cosmetic iris implants pose high risks of severe complications and are widely consid- ered unsafe. |
| Laser iris depigmentation is minimally invasive but lacks customization and robust long-term data. |
| Cosmetic keratopigmentation (KTP) is the safest and most effective method for perma- nent eye color change. |
| KTP offers customizable, durable results but requires careful patient selection, particularly avoiding patients who have undergone laser- assisted in situ keratomileusis (LASIK). |
| Further research is needed to enhance safety, outcomes, and pigment durability in these procedures. |
INTRODUCTION
The desire for cosmetic enhancement of eye color has driven innovation in ophthalmic sur- gery, resulting in techniques designed to alter the appearance of the iris. The approved proce- dures—laser iris depigmentation, and cosmetic keratopigmentation (KTP)—offer varying degrees of effectiveness and safety, but each comes with its unique benefits and challenges, while cos- metic iris implants are considered malpractice but are still performed in some countries [1].
While these interventions appeal to indi- viduals seeking aesthetic transformation, their adoption has sparked debate among ophthal- mologists because of risks associated with cer- tain methods. This review explores these tech- niques, their surgical underpinnings, clinical outcomes, and complications, offering a com- prehensive synthesis of current knowledge to guide clinicians and inform patients. This arti- cle is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
COSMETIC IRIS IMPLANTS
Cosmetic iris implants were originally devel- oped for congenital or traumatic iris defects [2], without any indications for implantation in eyes with clear crystalline lens and nor- mal iris. However, cosmetic iris implants have been repurposed for purely cosmetic reasons, to change iris color in healthy phakic eyes. A silicone-based prosthetic iris is inserted into the anterior chamber through a small limbal incision. Two popular models are designed to mimic natural iris pigmentation and patterns while anchoring securely within the eye: the NewColorIris (Kahn Medical Devices, US pat- ent 2006 #7025781 2B, available at https://ww. google.com/patents/US7025781) is a silicone iris diaphragm with six rounded flaps at the periph- ery designed to hold it in place, and is between
11.0 and 13.0 mm in diameter, with a pupillary
aperture of 3.5 mm and thickness of 0.16 mm; the BrightOcular (Stellar Devices LLC, US patent 2012 #8197540, available at http://www.google. com/patents/US8197540) presents some slight differences in size (11.5–13.5 mm in diameter and 0.16–0.18 mm in thickness). It is held in place by five peripheral triangular flaps, and its posterior face presents grooves to facilitate the flow of the aqueous humor [1].
Despite their technical sophistication, these implants have not received approval from major regulatory bodies, including the US Food and Drug Administration (FDA) or the European CE mark. Nevertheless, the technique has gained traction in regions with less stringent medical regulations, raising significant concerns within the ophthalmic community.
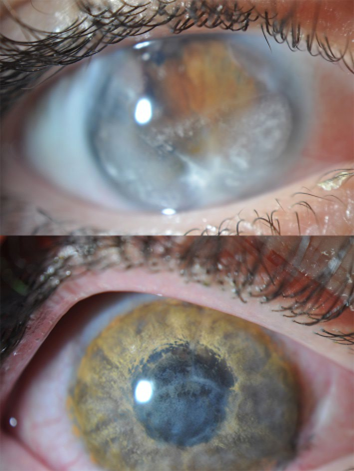
Reports on cosmetic iris implants consist- ently highlight their propensity for severe com- plications [3–6]. Early studies demonstrated that patients often develop anterior segment inflammation, corneal endothelial cell loss, and increased intraocular pressure (IOP), lead- ing to secondary glaucoma and even blindness in severe cases. A retrospective multicenter study of 65 eyes revealed that nearly 92% expe- rienced complications, ranging from corneal edema to cataracts, and 81% required implant removal within 2 years of surgery [6]. Even when explanted, residual damage—such as persistent endothelial cell loss or structural abnormali- ties—often necessitated additional interven- tions like corneal transplantation or glaucoma filtering surgery. In our series reported in 2021, we presented the outcomes of 10 eyes from five patients referred for the management of compli- cations following cosmetic iris implant proce- dures. Among these cases, two eyes had received NewColorIris implants, while eight had Bright- Ocular implants. All implants were ultimately removed because of severe postoperative com- plications occurring between 1 and 60 months after surgery. The mean endothelial cell density (ECD) was 848 ± 227.5 cells/mm2. Corneal trans- plantation was necessary in 30% of cases: two eyes underwent Descemet membrane endothe- lial keratoplasty (DMEK), and one eye received penetrating keratoplasty (PKP). Additionally, three patients were advised to undergo cor- neal transplantation (one PKP and two DMEK), although these procedures had not yet been per- formed. Ninety percent of eyes developed ocular hypertension or exhibited signs of glaucomatous optic neuropathy, with filtering surgery required in two cases (20%) to manage elevated IOP. Cataract formation was another frequent com- plication, occurring in 40% of patients. Among these cases, one patient required cataract surgery with implantation of a Morcher iris-intraocular lens (IOL) (Morcher GmbH, Stuttgart, Germany) because of severe iris atrophy. The mean age at the time of cataract surgery was 36 years [1].
Complications arise from both mechani-
cal and biochemical factors. Mechanically, the implant’s flanges and dimensions create tur- bulence within the anterior chamber, causing chronic endothelial trauma. Biochemically, low-grade inflammation induced by the foreign body exacerbates tissue damage over time. The risk of vision-threatening outcomes has led to a consensus among experts that cosmetic iris implants should be avoided and regarded as a form of malpractice.
Although the aesthetic appeal of cosmetic iris implants may initially satisfy some patients, the high likelihood of complications often results in dissatisfaction over the long term. For this reason, informed consent discussions should emphasize the procedure’s risks, including potential irreversible vision loss. Clinicians should counsel patients on alternative, safer methods, but be aware of their complications to treat them if patients have been referred for their resolution.
LASER IRIS DEPIGMENTATION
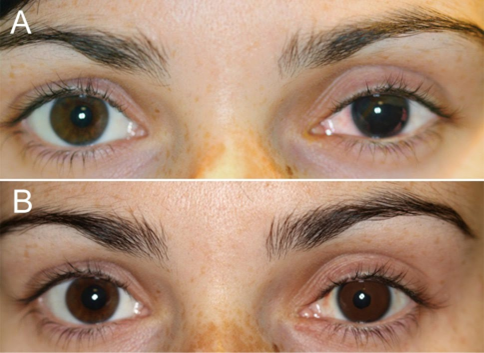
Laser iris depigmentation emerged as a less inva- sive alternative for cosmetic eye color change, leveraging technology from dermatologic laser treatments. The procedure employs utilizes a 532-nm Q-switched Crystal laser, deliver- ing 3–4 ns pulses, a technology commonly employed in selective laser trabeculoplasty, to selectively ablate melanin in the anterior iris stroma, revealing the underlying stromal fibers, which often appear blue or green. The number of treatment phases required to achieve the desired result depends on the pigment level and grade of the eye, with adjustments made as needed throughout the procedure. Each phase comprises four consecutive daily ses- sions treating both eyes, repeated at intervals of 4–6 months until completion. Unlike cosmetic implants, this method does not introduce for- eign materials into the eye, positioning it as an appealing outpatient option.
Initial investigations into laser depigmenta-
tion were inspired by treatments for oculoder- mal melanocytosis and cutaneous pigmenta- tion [7]. The method was subsequently adapted for cosmetic purposes, with the potential to achieve lasting results without surgical incisions. While the laser treatment itself is painless, some patients may experience mild discomfort from the slit-lamp’s bright light. Although laser iri- doplasty offers several advantages, it may cause certain mild and short-term complications, including secondary hypertension (a temporary and slight increase in IOP caused by a sudden, partial obstruction in the drainage of aqueous humor), acute depot (a condition characterized by reduced pigmentation deposits on the lower iris, which typically fade over time, although minimal residual pigmentation may persist), microhemorrhage (small bleeds within the stro- mal tissue that resolve spontaneously within seconds as a result of natural ocular pressure), and acute iritis (an inflammation that may result in eye redness, discomfort, tenderness, and sen- sitivity to light).
The largest prospective study to date evalu- ated 1176 eyes undergoing laser iris depigmen- tation, reporting high patient satisfaction and effective melanin removal [8]. Complications were relatively mild, with transient iritis occur- ring in 25% of cases and resolving with topical anti-inflammatory therapy. However, isolated reports have documented more serious out- comes, including stromal atrophy, chronic pho- tophobia and iatrogenic pigmentary glaucoma [1, 9], emphasizing the need for careful patient selection and technique refinement.
Experimental animal models have highlighted additional risks [10]. Rabbits treated with vary- ing laser energies exhibited patchy depigmen- tation and hyperpigmented granules in the iris stroma. Moreover, some developed mild anterior chamber inflammation, underscoring the impor- tance of optimizing laser parameters.
Despite its promise, the technique remains investigational in many regions as result of sparse long-term data. Furthermore, its aesthetic limitations—specifically, the inability to achieve customized colors—may leave some patients dissatisfied.
COSMETIC KERATOPIGMENTATION
Cosmetic keratopigmentation (KTP) represents the most rigorously studied method for chang- ing eye color, since the first seven patient treated worldwide for purely cosmetic KTP in 2014 [11]. Rooted in ancient corneal tattooing techniques, Galen (131–210 AD) is considered to be the first one to have used pigments for human cornea, using reduced copper sulfate to mask a corneal leukoma. Alio and his research team have been the first to describe in the scientific literature the modern techniques of therapeutic and cos- metic KTP, offering unparalleled precision and safety with the advent of femtosecond lasers and biocompatible pigments [1, 11–23]. Alio et al. described several techniques to perform KTP, tailored to its use for purely cosmetic purposes but especially to be used in the different eye cosmetic morbidities that are shown by differ- ent types of patients for therapeutic indications, including corneal opacities that may be super- ficial, deep, or a combination of both, as well as other visual impairments, such as absolute glaucoma, squint, ptosis, and enophthalmos. The introduction by Alio and co-workers of automated devices has largely replaced manual procedures, though these traditional methods are still occasionally used in specific cases.
Today, KTP techniques are generally classified into three categories: superficial, intrastromal, and mixed [19].
The manual intralamellar KTP method uti- lizes a diamond knife to create incisions reaching approximately 40–50% of the corneal thickness, as measured by pachymetry. A specialized set of helicoidal dissectors is then used to form circular intrastromal tun- nels.
Lastly, femtosecond laser-assisted kerato- pigmentation (FIK) enables the precise crea- tion of multiple, uniform tunnels at vary- ing depths and dimensions, customized according to the surgeon’s expertise and the specific requirements of each case. Tunnel depths usually ranged from 300 to 350 µm from the corneal surface, with an inner pupil diameter of 5.5 mm and an outer diameter of
9.5 mm, with a vertical incision positioned at 6 o’clock. A lamellar dissector is then used to open and expand the femtosecond laser- created tunnel to its external corneal limits, extending toward the limbus (Fig. 2).
The modern KTP procedure involves depos- iting micronized mineral pigments into the corneal stroma [17]. Customized micronized mineral pigments were prepared to match the patient’s desired color, with test applica- tions performed in a wet lab prior to surgery to ensure proper color matching. During the procedure, the pigments were introduced into the deeper tunnel using a 27-gauge cannula via the 6 o’clock incision. Additionally, minor refinements were performed using the super- ficial technique. These pigments, certified for biocompatibility under European cosmetic regulations, are customized to achieve a range of natural colors, and even combined together is necessary. Advanced digital simulations are often used preoperatively to align patient expec- tations with achievable outcomes.
KTP has demonstrated excellent safety and efficacy in both therapeutic and cosmetic con- texts. After its first application in a case of essen- tial iris atrophy [11], cosmetic KTP has been largely studied: in 2021, a prospective study involving 79 eyes reported stable pigmenta- tion patterns and high patient satisfaction, with no significant impact on visual acuity or corneal integrity during a follow-up period of up to 69 months [13]. Safety and outcomes of therapeutic cosmetic KTP have been lately con- firmed in another series of 85 patients which underwent femtosecond laser-assisted intras- tromal and superficial automated KTP (FIK and SAK) using micronized mineral pigments: excel- lent or good cosmetic outcome was reported by the independent observer in 91.4% of the SAK group, 91.5% of the FIK group, and 100% of the combined SAK + FIK group [24].
Alio et al. studied and reported the outcomes
of 234 eyes that received therapeutic and KTP with various techniques and reported the com- plication rate as 12.82% [15]. The most common complication of their study was light sensitiv- ity (49% of complicated eyes), whereas the least common ones were visual field limitation and magnetic resonance imaging (MRI) alterations which occurred in 4% and 2% of complicated eyes, respectively. Other reported complications were color fading, change in color (both 19% of complicated eyes), and neovascularization (7% of complicated eyes).
In contrast to laser iris depigmentation, KTP offers greater flexibility in achieving precise colors and patterns, allowing patients to tailor their appearance to their preferences (Fig. 3). This customization, combined with its excellent safety profile, positions KTP as the gold stand- ard for cosmetic eye color change, and the long follow-up of therapeutic cases demonstrated its safety. Developments in micronized mineral pig- ments might be implemented and completed by the industry in the future to provide pigments of different densities, different colors, free from iron and metals that may induce redox reac- tions, and more specifically designed for thera- peutic or purely cosmetic indications.
DISCUSSION
The diverse approaches to cosmetic eye color change reflect the intersection of aesthetic
demand and surgical innovation. While previous publications by our group have addressed gen- eral or therapeutic uses of eye color change tech- niques, this review provides a focused, updated synthesis of purely cosmetic surgical approaches. It aims to clarify the relative advantages, risks, and limitations of each method using the most recent evidence, thereby guiding clinicians and informing patients considering aesthetic eye color change. Despite the common goal of the different surgical procedures, these methods dif- fer significantly in safety, efficacy, and patient satisfaction (Table 1).
Laser iris depigmentation offers a minimally invasive option, performed in outpatient set- tings using Nd:YAG lasers, to reduce melanin on the anterior iris surface. This technique can reveal underlying natural stromal fibers, such as blue or green, in individuals with darker irises. While the procedure promises a natural and subtle transformation, it comes with nota- ble constraints. Patients cannot select a specific desired eye color, as the outcome is dictated by the underlying natural pigmentation. Further- more, the technique remains in an experimental phase, with limited long-term studies to validate its safety and effectiveness. Reports of patchy pigmentation, hyperpigmentation, and mild anterior chamber reactions have emerged, albeit typically resolving in the short term. Laser iris depigmentation is best suited for patients will- ing to accept these uncertainties and limitations, understanding that the results may not meet exact expectations.
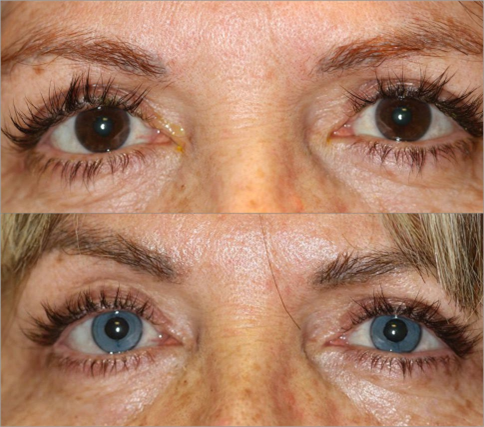
Table 1 Comparative analysis of techniques
| Technique | Safety | Effectiveness | Patient satisfaction | Regulation |
| Cosmetic iris implants | High risk of complica- tions, including glau- coma and endothelial damage | Immediate but high complication rate | Low due to long-term risks and severe visual loss | Not approved by FDA or CE |
| Laser iris depigmenta- | Relatively safe; lacks | Effective for lighter | Moderate; aesthetic | Investigational in |
| tion | long-term data | irises; limited for | limitations | many regions |
| Cosmetic keratopig- | Minimal risks with | darker irises Highly effective and | High due to durability | Approved in several |
| mentation | proper screening | customizable | and personalization | regions |
CE Conformité Européenne, FDA US Food and Drug Administration
Cosmetic iris implants, in contrast, provide dramatic and immediate eye color changes with a wide variety of shades to choose from. How- ever, the allure of these implants is overshad- owed by the significant risks they pose. Neither FDA-approved nor CE-marked, these devices have been linked to severe and often irrevers- ible complications, including glaucoma, uveitis, corneal endothelial damage, and vision loss. The implantation process itself can trigger a cascade of issues requiring extensive corrective surgeries, such as corneal transplants or implant removal. Additionally, patients frequently report inade- quate postoperative care, exacerbating the risks. As such, cosmetic iris implants are not a recom- mended option. Their use is considered a mal- practice risk, and patients seeking this procedure should be strongly discouraged and redirected to safer alternatives.
KTP, as firstly described by Alio et al. [11],
stands out as the most promising and well- researched option among the three techniques. With roots in traditional corneal tattooing, KTP has evolved into a sophisticated and precise pro- cedure that delivers stable, predictable results. By implanting customized mineral pigments into the corneal stroma, this method allows patients to achieve a natural and aesthetically pleasing
change in eye color. Extensive studies have dem- onstrated KTP’s safety, with minimal impact on visual acuity or corneal anatomy. While some patients may experience temporary light sensi- tivity, advancements in technique, such as deep- ening the stromal pocket, have largely addressed this issue. KTP has also achieved high rates of patient satisfaction, making it the most reliable option for those seeking a permanent cosmetic change (Fig. 4). However, it is important to note that patients with a history of LASIK surgery are generally not suitable candidates because of the potential for complications, whereas those treated with photorefractive keratectomy (PRK) are considered viable. As cosmetic KTP is often performed in young, healthy individuals, it is important to consider how this interven- tion may influence the future management of age-related ocular diseases. Theoretically, the presence of corneal pigments could affect visu- alization during anterior or posterior segment procedures such as cataract surgery, retinal detachment repair, or macular surgery. How- ever, a recent publication by Ferrari et al. [25] demonstrated that all standard ocular exami- nations can be effectively performed through a keratopigmented cornea, except for gonioscopy, which may be compromised because of pigment obstruction of the iridocorneal angle. While there is no direct clinical evidence regarding IOL power calculation through keratopigmented eyes, theoretical considerations and the preser- vation of a clear central corneal zone suggest that biometry and IOL calculation should not be affected. That said, caution and individualized assessment remain essential. Future therapeutic interventions that target the anterior chamber angle—such as many modern minimally inva- sive glaucoma surgeries (MIGS) procedures— may be hindered or contraindicated following KTP, and this potential limitation should be dis- cussed with patients as part of informed consent. Although KTP appears to be the safest option for cosmetic eye color change on the basis of the data currently available, this conclusion is primarily drawn from smaller, single-center studies. As such, the evidence remains limited in scope and generalizability. To establish a more definitive understanding of its safety and effi- cacy, future research should involve larger, mul- ticenter clinical trials with robust methodology and long-term follow-up. This will help ensure that the initial findings are reliable and applica- ble across broader populations.

The regulatory landscape surrounding cos-
metic eye color change procedures remains fragmented. While KTP is performed legally in several countries under off-label use or institu- tional protocols, other techniques—particularly cosmetic iris implants—are explicitly discour- aged or unapproved by regulatory agencies such as the FDA and CE. Ethical concerns include informed consent, the irreversible nature of
some interventions, and the balance between cosmetic benefit and surgical risk in otherwise healthy individuals. At present, there is no for- mal international consensus or guideline on these procedures. We believe that increased collaboration among ophthalmic societies and regulatory bodies is essential to establish safety standards, define ethical boundaries, and ensure patient protection.
CONCLUSION
For individuals considering these procedures, the choice ultimately hinges on balancing risks and expectations. While KTP offers a safe and effective path for most patients, laser iris depig- mentation might appeal to those willing to navigate its experimental nature. In contrast, cosmetic iris implants should be unequivo- cally avoided because of their dangerous and potentially devastating complications. Com- prehensive preoperative counseling and tailored postoperative care are crucial to ensure patient satisfaction and minimize risks. The decision to alter one’s eye color is deeply personal, but it must be grounded in a clear understanding of the medical implications and aesthetic possibili- ties of each technique. Late-onset complications remain a concern and procedures such as KTP must be evaluated in the context of lifelong ocu- lar health.
Author Contribution. Francesco D’Oria and Jorge L Alio contributed to conceptualization, methodology and validation, data curation, writing—original draft preparation, review and editing. All authors have read and agreed to the published version of the manuscript.
Funding. No funding or sponsorship was received for this study or publication of this article.
Data Availability. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of Interest. Jorge L. Alio is an Editorial Board member of Ophthalmology and Therapy. Francesco D’Oria is an Advisory Board member of Ophthalmology and Therapy. Franc- esco D’Oria and Jorge L. Alio were not involved in the selection of peer reviewers for the man- uscript nor any of the subsequent editorial decisions.
Ethical Approval. This article is based on previously conducted studies and does not con- tain any new studies with human participants or animals performed by any of the authors. Per- mission has been received from the patients for publication of their images.Open Access. This article is licensed under a Creative Commons Attribution-NonCommer- cial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, pro- vide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons
licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the cop- yright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
